

- Percent error chemistry calculator how to#
- Percent error chemistry calculator plus#
- Percent error chemistry calculator free#
Percent error chemistry calculator free#
It is a free online tool that allows you to perform many experimental measurements.The percent calculator can be used in chemistry to measure the accuracy of the chemical experiment.When doing some experimental analysis with different tools like Vernier calipers or a screw gauge, you can use it.It provides you with quick and accurate results because it is an efficient tool made to execute faster and correctly.It gives you the actual error percentage of the measurements to fully trust it.So, there are many benefits of using this tool. This error tool calculates the percentage of the difference between real and measured values. Benefits of using Percentage Error Formula Calculator It can only do it by using this error finder tool. The percent error formula can be used to know the difference between the actual and expected results. In science, there are many theoretical ideas near the actual results. There is always a need to use a tool to compute it accurately. Since the percent error formula is used to measure the difference between experimental and theoretical values in percentage taken from an instrument. Now enter the actual value in the “Observed value”.Īs you click on the calculate button, you will find the results within a few seconds.In the first step, you need to enter the observed value in the “True value” box.There are some simple steps to using this tool.
Percent error chemistry calculator how to#
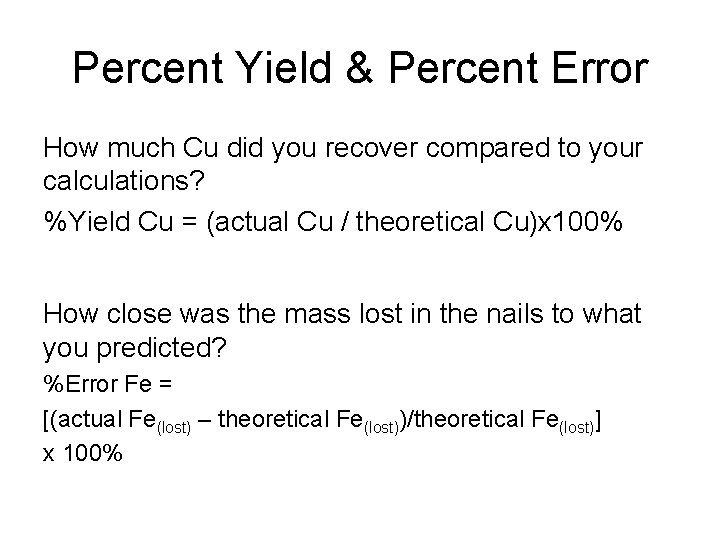
How to use the Percent Error Calculator with steps? $$ Percent \ Error \ =\ \frac \ ×\ 100 $$ $$ Percent \ Error \ =\ 0.11×100 $$ $$ Percent \ Error \ =\ 11% $$Ĭalculatores offers you many other tools like limiting calculator to calculate rate of change of function, logarithm calculator to find log of any number and anti-log calculator to find antilog. The following formula is used to calculate it, Percentage error is the difference between actual value and the observed value in comparison to the actual value. Formula used by Online Percent Error Calculator Related:You can also use sig fig calculator to round significant figures and salvage value calculator to calculate salvage value of any object. That’s why here we introduce a tool online that can easily find percentage error. You always need a tool that can find percentage error to find the difference between exact and observed value. When you are doing approximations using different gadgets, there are many chances of getting errors in observations. You can use it for numerical approximations as well as in science to report errors in observations. It used to calculate percent error in approximations. It uses a percentage error formula to find percent error.
The table shows five measurements for the volume of acid required in a neutralisation reaction.Ĭalculate the mean volume and estimate the uncertainty.The percentage error calculator is an online tool that calculates the percentage of error between the observed and exact values of measurements. This means that the value can be given as the mean value ± half the range. Estimating uncertainty from sets of repeat measurementsįor a set of repeat measurements, the uncertainty is ± half the range. For a timer reading to 0.1 s, the uncertainty is ± 0.05 s. This means that if a student reads a value from this thermometer as 24.0☌, they could give the result as 24.0☌ ± 0.5☌.įor a digital measuring instrument, the uncertainty is half the last digit shown on its display.

For a thermometer with a mark at every 1.0☌, the uncertainty is ± 0.5☌.
Percent error chemistry calculator plus#
The uncertainty of a measuring instrument is estimated as plus or minus (±) half the smallest scale division. It has a higher resolution than a thermometer with a mark at every 2.0☌. A thermometer with a mark at every 1.0☌ has a resolution of 1.0☌. The resolution of a measuring instrument is the smallest change in a quantity that gives a change in the reading that can be seen. from the range of a set of repeat measurementsĮstimating uncertainty from measuring instruments.by considering the resolution of measuring instruments.There are two ways of estimating uncertainty: exactly when a chemical reaction has finished.whether a thermometer is showing a temperature of 24.0☌, 24.5☌ or 25.0☌.

For example it may be difficult to judge: There are many causes of uncertainty in chemical measurements. Whenever a measurement is made in chemistry, there is always some uncertainty in the result obtained.


 0 kommentar(er)
0 kommentar(er)
